Draw the two structures in stereoscopic projection with dash wedge etc. Newman projection was named after the American chemist Melvin Spencer Newman.

Toluene Activator Ortho Para Director Aromatic Substitution Mechanism Organic Chemistry Books Chemistry Organic Chemistry
For chair cyclohexane imagine looking down the two bonds that form the seat of the chair C2-C3 and C5-C6.

. Just know how to draw the newman projections for the molecules talked about in lecture. 6 Drawing Chair Conformations 1. Connect the tops with a V 3.
Basically all we have to do is create two separate Newman projections and link them together through two different carbons. Technology to the rescue. 2 Draw first carbon in the bond as a dot.
I feel your pain so I created some interactive models for you below. And then the blue carbon is going to be in the back. And in the back you have this blue one.
Energy diagrams show the relative energy of a molecule compared to rotation about the axis of interest. The lowest energy conformation is to arrange the substituents of the two carbons in the anti configuration places Cl and Br opposite of each other. The blue carbon is in.
Butane C2-C3 front carbon is C2 STERICS 3 b. Molecule being drawn in the Newman Projection is 2-butanol. Looking down the barrel of the sp3 bond we place carbon 1 substituents in the front in red and carbon 2 substituents in the back black.
We actually use what amounts to two Newman projections stuck together and we call it a double Newman. Use Orbital Tool to draw Newman projection circle. Draw the lowest energy conformations of the molecules shown below and identify all the gauche-butane interactions in these molecules.
4 Draw atoms around the first carbon. For cyclic compounds draw both chair conformations of the molecule. You can draw Newman projections for cyclic molecules.
When drawing Newman projections look at the molecule from a. Butane C2-C3 front carbon is C2 TORSIONAL. Draw the energy diagram for a Newman projection.
Newman projections focus on any two carbons and the groups coming off them in a molecule by shifting the view from which the molecule is visualized. You can draw them close together just connect both of them to the top CH2 the back of the chair and the bottom CH2 bottom of the chair. Label each hydrogen as H1 H2 etc.
Draw side-by-side projections of the C1-C2 and C5-C4 bonds. Draw the Fisher projection of the molecule. On one of the models switch two of the balls.
-Cl is smaller than any alkyl group a. A Newman projection is a way to take a snapshot of what a molecule looks like at a particular moment in time from a different angle than were used to. Draw 2 parallel slanted bonds.
Generally these start at 0 degrees and rotate through the entire molecule. Notice that the pairs of equatorial bonds are parallel to pairs of nonadjacent ring bonds. Two perspectives are commonly used.
3 Draw second carbon in the bond as a circle surrounding the dot. This can then be graphed showing which parts and bond angles about the axis of interest are more or less stable. Draw its most stable Newman projection about the C3-C4 bond.
Make two identical models of methane with four different colored balls attached to each carbon. While not a stereocenter drawing the hydrogens in this way makes drawing the Newman Projection much easier. The arrow goes counterclockwise indicating S configuration and this means in the original molecule it is R.
Add the groups here they are H atoms to the bonds extending from the Newman projections. This tutorial demonstrates how to draw a Newman projection in ChemDraw using ethane as our example molecule. Draw Newman projection for the molecule below from the perspective indicated.
Newman Projections Ring Strain. You can check your answers by expanding the tab below each question. Draw methane half of ethanes structure Duplicate the methane and draw bond between both.
For each of the following determine what strain energy is involved in each Newman projection torsional andor steric to explain why the first Newman projection is more stable than the second. You can imagine in the front if we want to maybe Ill do a little small orange thing to show this is the orange carbon. For the cyclic compounds your Newman projection should be looking down the C1-C2 and C4-C5 bonds.
Rotate one methyl moiety by 180. Lets draw one for 1R2R4S-4-chloro-2-iodo-1-methylcyclohexane. Join the two diagrams with the front carbon C-6 at the top and the rear carbon C-3 at the bottom.
Both of these have gauche conformations. For the Newman projection you drew indicate if there are any eclipsed bonds or gauche butane interactions. Fischer projections are just another way of drawing compounds contacting chirality centers.
Grab a pen and some paper and use the model to draw Newman projections for each of these from the point of view indicated by the beady little eye. The sawhorse projection is described below. For this we utilize the Newman projection.
They were initially proposed by Emil Fischer for making it easier to draw the structures of compounds containing multiple chirality centers with the main idea of not having to draw the wedge and dash lines for every single chiral centerThis is especially applicable and used mostly for drawing. The Natta projection is a type of projection that we can use to depict molecules in a complete stereochemistry in 2D skeletal formula. Connect the bottoms with an inverted V 7 Drawing Chair Conformations Every carbon also has an equatorial bond.
For the boat form repeat the process. 1-Chloropropane Saturated Cyclic Compounds Cyclopropane Angle and Torsional Strain Electron Density Map All Dihedral Angles 0o Cyclobutane is not Planar Cyclopentane Cyclohexane Chair Conformation link to active site Boat Conformation How to Draw a Chair Conformation all opposite bonds are parrallel Axial. Alternatively which is more time-consuming you can draw the Newman projection of the molecule looking from the angle that places group 4 in the back pointing away from the viewer.
So just worry about drawing the newman projection for Ethane including different substituents on ethane and how the different substituents affect the total energy of the molecule depending on the conformation. He discovered this structure in 1952 as a partial replacement of the Fischer projection.

Ring Flip Comparing The Stability Of Chair Conformations With Practice Problems Chemistry Steps
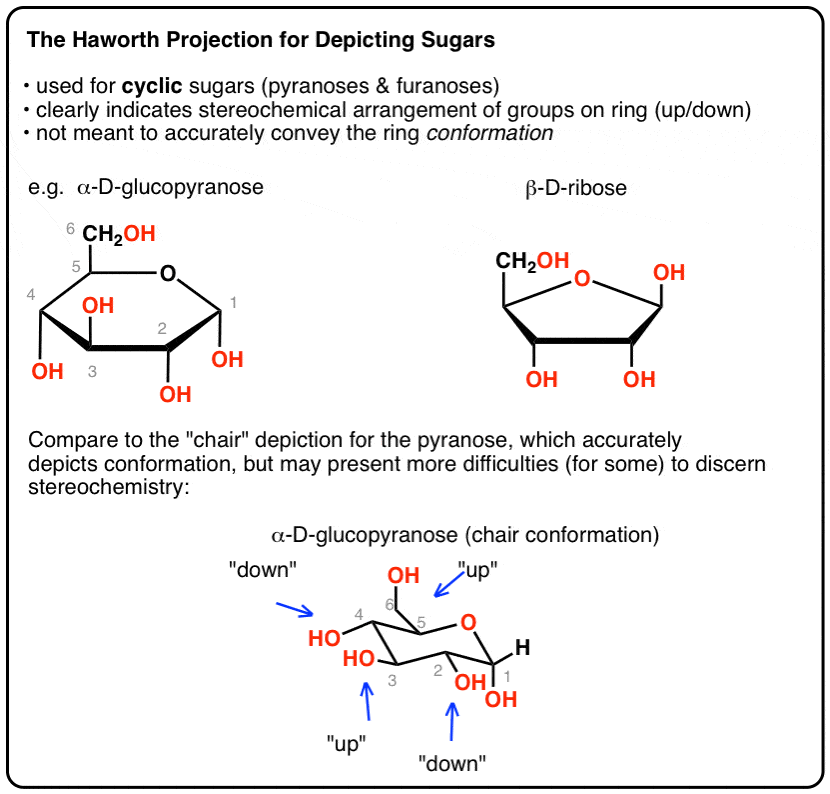
The Haworth Projection Master Organic Chemistry
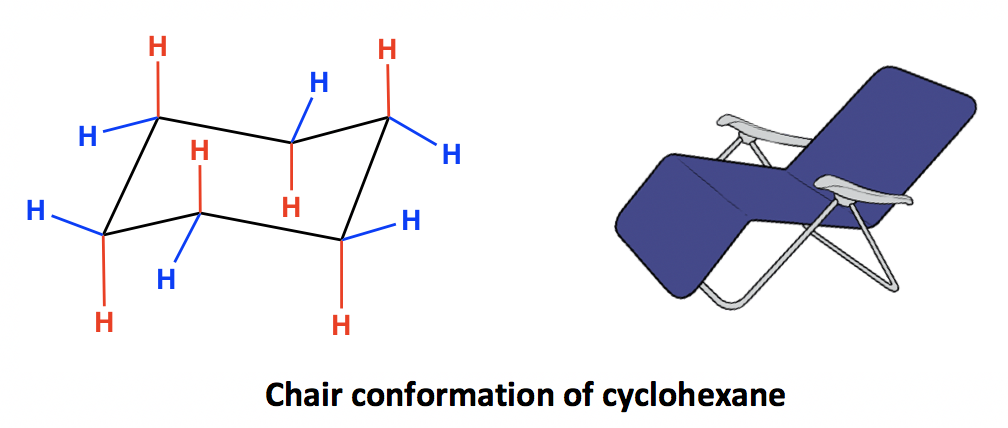
4 3 Conformation Analysis Of Cyclohexane Organic Chemistry I

Organic Chemistry Conformation Of Cyclic Alkanes Part 1 3 Youtube
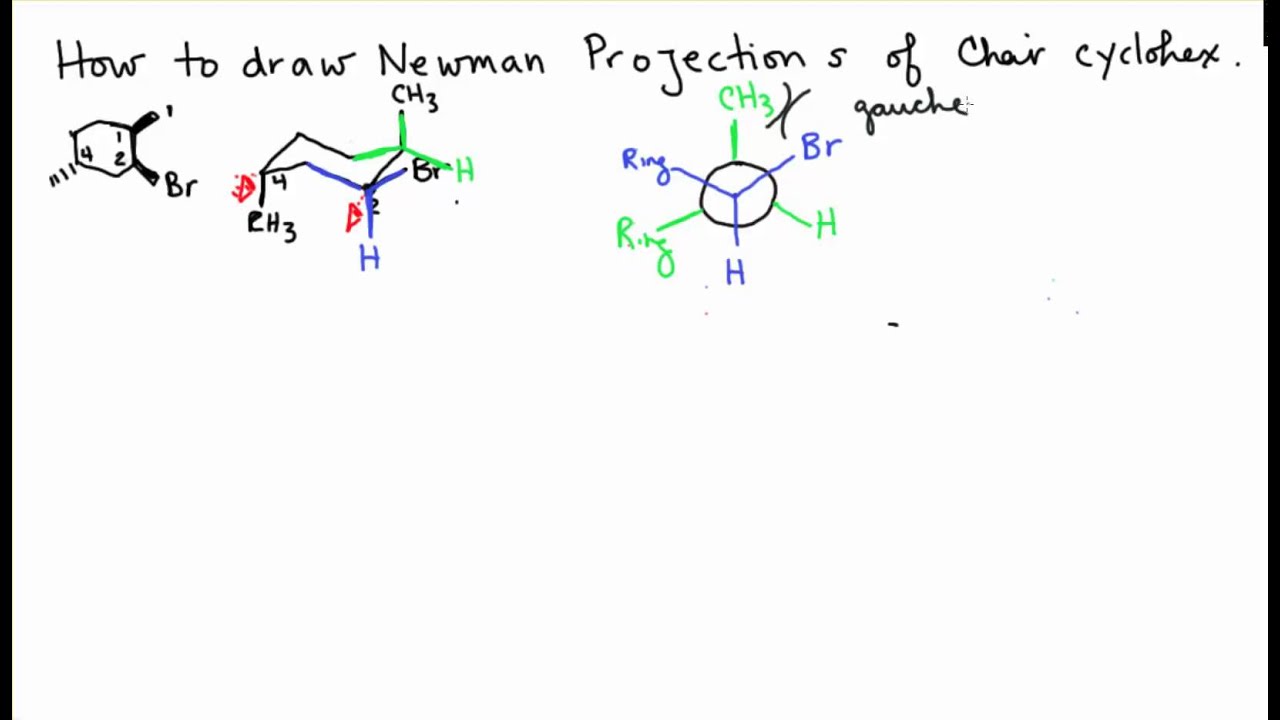
Double Newman Projection Chair Cyclohexane Youtube

Solved Part Ii Conformations Of Cyclic Compounds A Chegg Com

Cyclohexane Chair Conformation To Double Newman Projection Youtube

Ring Flip Of Chair Conformations With Practice Problems Chemistry Steps

Newman Projection Task And Its Solution Download Scientific Diagram

Pin On Alkene Reactions With Practice Problems

How To Draw Cyclohexane Chair Conformations And Ring Flips Youtube
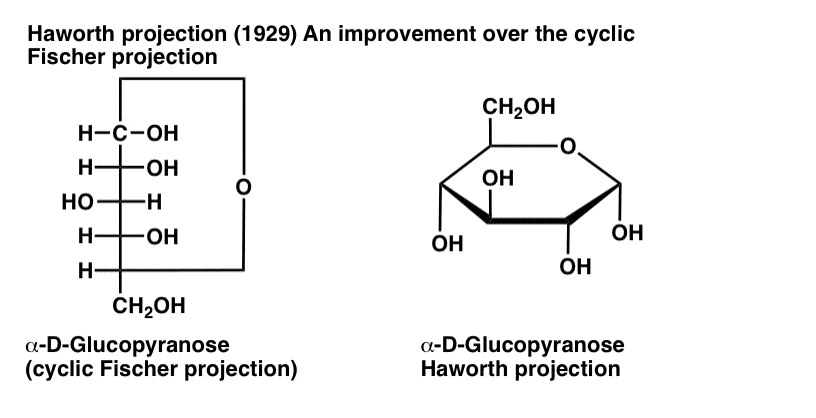
The Haworth Projection Master Organic Chemistry

Newman Projection Of Butane Learn How To Analyze The Staggered And Eclipsed Conformatio Organic Chemistry Organic Chemistry Study Organic Chemistry Reactions

Intro To Orgo 1 Of 5 Atoms Atomic Structure Periodic Table And Trends Periodic Table Atomic Structure Intro

Conformational Analysis Newman Projections Ring Strain Cyclohexane Conformations Ppt Download



